Abstract
BACKGROUND: Over the last 40 years, there has been a gradual improvement in outcomes in acute myeloid leukemia (AML), yet AML remains a highly aggressive malignancy responsible for over 11,000 deaths per year in the United States and a 5-year overall survival of 30%. Recent advancements in the management of AML include hypomethylating agents, venetoclax, and targeted therapy such as FLT3 and IDH inhibitors. However, treatment outcomes and survival rates in clinical trials may not correlate with real-world experience as clinical trials may under-represent individuals with organ dysfunction, decreased performance status, and older age. There is a particular paucity of data regarding AML management and outcomes in a safety net hospital setting such as Harbor-UCLA. Herein, we report a retrospective cohort of AML patients to evaluate patient characteristics, management patterns, and outcomes in comparison to national data and trends.
METHODS: We performed a retrospective chart review of all patients aged 18 years or older who presented to Harbor-UCLA Medical Center between 2008-2022 with the diagnosis of AML. The diagnosis of AML was based on the presence of 20% or greater myeloid blasts in the peripheral blood or bone marrow or recurrent genetic abnormalities including t(8;21), inv(16), or t(16;16). Patients with acute promyelocytic leukemia were excluded. Data collected included demographics, disease characteristics, baseline laboratory values, treatment, and outcomes.
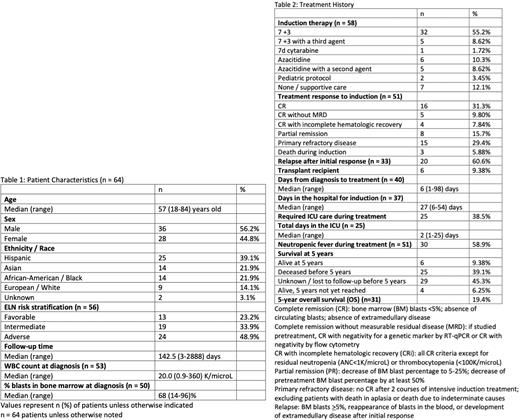
RESULTS: We identified a total of 64 patients with AML. The median age at diagnosis was 57 years old (range 18-84 years old), and the cohort was 39% Hispanic, 22% Asian, 22% Black, 14% European, and 3% unknown. In regards to European LeukemiaNet (ELN) 2017 risk stratification, 23%, 34%, and 49% of patients had favorable, intermediate, and adverse risk AML, respectively. Thirteen patients (20%) had FLT3 mutations, and 5 patients (8%) had IDH1/2 mutations. The cohort included 6 patients (9%) with therapy-related AML and 16 patients (25%) with AML with myelodysplastic changes. Induction regimens included 7+3 (55%), 7+3 with a third agent (9%), azacitidine (10%), and azacitidine with a second agent (9%). Seven patients (12%) received only supportive care. The median time from diagnosis to treatment was 6 days, and the median time spent in the hospital for induction was 27 days. The complete remission rate (CRR) to induction therapy was 48.9% with 21 patients (41%) achieving complete remission (CR) and 4 patients (8%) achieving complete remission with incomplete hematologic recovery (CRi). Three patients (6%) died during induction. Of the patients with initial response to treatment, 61% later relapsed. Six patients (9%) received allogenic stem cell transplant. Five-year overall survival was 19.4%.
CONCLUSIONS: In this diverse population of patients with no or suboptimal insurance, the plurality of which consists of adverse risk AML (49%), we observed a complete remission rate of 48.9% to induction therapy and a 5-year overall survival of 19.4%, rates which are slightly lower than clinical trial and SEER data. A relatively low rate of allogeneic stem cell transplant (9%) was observed, which may contribute to poor 5-year overall survival. Further research is needed to characterize real-world AML outcomes and elucidate potential mechanisms of disparity.
Disclosures
Tomassetti:Novartis: Research Funding; Beigene: Research Funding; Rigel: Research Funding; SeaGene: Research Funding; Merck: Research Funding; Principia: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal